A surgical site infection (SSI) is a post-surgical infection that can affect either the incision or deep tissue at the operation site. They have negative impacts on patients and hospitals, increasing the average patient’s hospital stay by approximately 6.5 days1. The typical cost of treating an SSI can range between $12,0002 and $125,0003 per patient. Leukomed® by Leukoplast® is a range of dressings and other solutions to help to minimize the risk of SSIs.
Safeguard your patient’s surgery
Leukoplast® helps to protect your surgical results from surgical site infections

We help you minimize the risks of SSIs
Leukomed® by Leukoplast® is our solution to support the prevention of SSIs. A range of post-surgical dressings for use in multiple post-operative situations. We also offer a range of frameworks, guidelines, and support tools to assist you in minimizing the risk of SSIs, as well as ongoing access to our dedicated representatives.
Up to 14 days’ wear time with Leukomed® Control
Leukomed® Control allows for easy wound monitoring without removal, preventing unnecessary dressing changes. It protects against water, bacteria, viruses, and contaminants, and conforms well even to contoured body areas9.
Proven results with Leukomed® Sorbact®
The surface of Sorbact® irreversibly binds, inhibits, and removes bacteria through a physical mode of action and it is safely removed4,5. This leads to an effective reduction of the bacterial burden in critically colonized or locally infected wounds6. Treatment resistance of hydrophobic microbes is not expected. Clinical evidence shows that Leukomed® Sorbact® delivers results.
- 65% relative risk reduction of acquiring an SSI7
- 57% cost reduction when treating caesarian sections8
Discover our range of surgical site dressings
All Leukomed® surgical site dressings
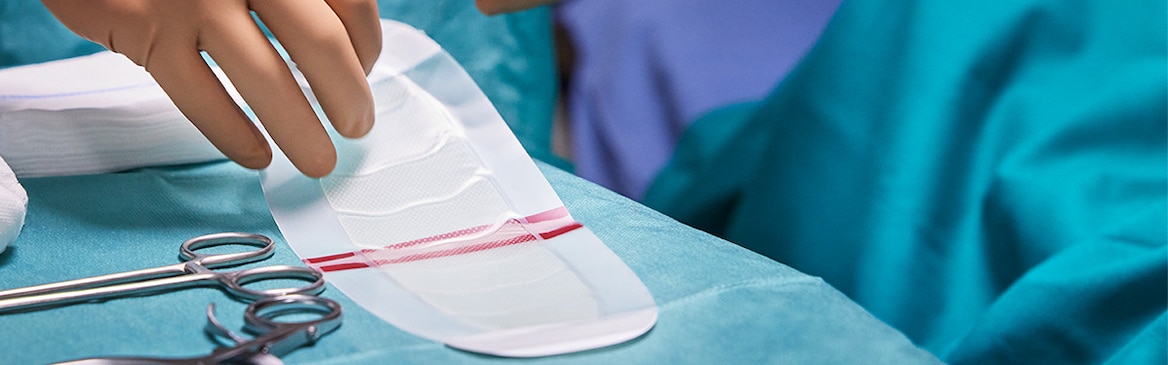
Applying Leukomed® Sorbact®
Discover the correct way to apply the dressing to a wound to ensure the best possible results for your patient.
Discover Leukomed® Control
Explore the benefits of Leukomed® Control, our transparent dressing for treating dry and low-exuding wounds.

1 Global guidelines for the prevention of surgical site infection, second edition ISBN 978-92-4-155047-5 © World Health Organization 2018
2 Tanner J, et al. Post discharge surveillance to identify colorectal surgical site infection rates and related costs. J Hosp Infect 2009;72:243e50
3 Getting it Right First Time, SSI National Survey, April 2019, https://gettingitrightfirsttime.co.uk/wp-content/uploads/2017/08/SSI-Report-GIRFT-APRIL19e-FINAL.pdf)
4 Husmark J, et al. Antimicrobial effects of bacterial binding to a dialkylcarbamoyl chloride-coated wound dressing: an in vitro study. J Wound Care. 2022 Jul 2;31(7):560-570.]
5 Ljungh, et al. Using the principle of hydrophobic interaction to bind and remove wound bacteria, Journal of Wound Care, Vol 15, No 4, 2006.
6 Mosti G, et al. Comparative study of two antimicrobial dressings in infected leg ulcers: a pilot study. J Wound Care. 2015;24(3):121-127.
7 Stanirowski PJ, et al. (2016b) Randomized controlled trial evaluating dialkylcarbomyl chloride impregnated dressings for the prevention of surgical site infections in adult women undergoing caesarean section. Surg Infect (Larchmt), 17(4): 427-35, 2016.
8 Stanirowski PJ, et al. Cost-effectiveness of a bacterial-binding dressing to prevent surgical site infection following caesarean section. J Wound Care. 2019 Apr 2;28(4):222-228.
9 Rousseau T, et al. An advanced transparent hydropolymer wound dressing for undisturbed post-op management of surgical wounds following hip and knee replacement: A prospective observational series; Int Wound J. 2021;1–7


